Internal vs. External GMP Audits: Key Differences Explained
Compliance is never optional in the pharmaceutical and biotechnology sectors. Any product reaching patients has to be up to the highest standards of quality and safety, and regulators expect companies to deliver on every step of the way. That is why GMP Auditing Services are an essential part of maintaining organizations in compliance, dependability, and trustworthiness.
Among the numerous forms of audits, two are imperative: internal GMP audits and external GMP audits. Both have the same purpose of ensuring compliance with Good Manufacturing Practice standards but differ in intention, operation, and repercussions.
For patient safety, supply chain integrity, and regulatory compliance companies, such knowledge may be the difference between seamless operations and expensive fines.
This post deconstructs internal vs. external GMP audits in detail. We’ll discuss what each entails, the benefits of each, typical challenges, and why blending the two provides a more powerful quality platform.
What Are GMP Audits and Why Do They Matter?
The foundation of quality in the biotechnology, pharmaceutical, and associated industries is Good Manufacturing Practice (GMP). It ensures that medications are safe, effective, and produced under uniform conditions by encompassing all stages of production, distribution, and control.
GMP compliance is tracked and improved through audits. Audits make procedures more transparent, point out any weaknesses, and make sure businesses are meeting legal requirements. Without them, there is a much greater risk of tainted goods, recalls, or improper inspections.
Audits are more than just a regulatory checkbox exercise for quality and compliance professionals. They are strategic instruments that reinforce systems, minimize risk, and keep patients safe. And with the help of expert GMP Auditing Services, audits can even introduce outside expertise that challenges organizations to rise to the highest standards.
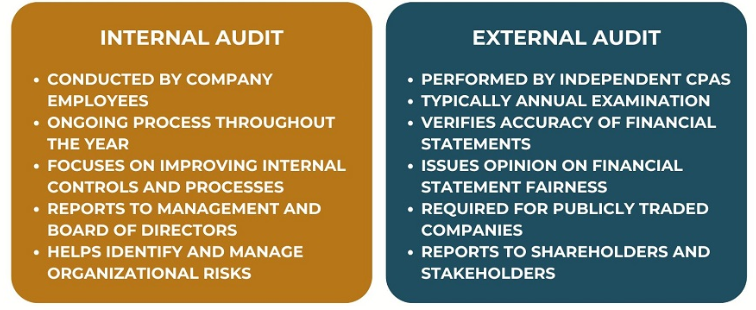
Internal GMP Audits: A Closer Look
Internal audits are self-checks carried out by a company on its own processes. They are sometimes referred to as self-inspections and are typically organized by the Quality Assurance (QA) department.
Objectives of Internal Audits:
- To detect weaknesses in processes before regulators do.
- To verify proper adherence to standard operating procedures, or SOPs.
- To guarantee preparedness for examinations by organizations such as the EMA or MHRA.
- To promote ongoing development as opposed to band-aid solutions.
Who Conducts Internal Audits:
- In-house QA teams.
- Cross-functional staff trained to perform audits.
- Independent consultants brought in for an objective view.
Benefits of Internal GMP Audits:
- Early detection of compliance risks.
- Stronger quality culture across teams.
- The capacity to adequately get ready for outside inspections.
- Chances for preventative and remedial measures (CAPAs).
Internal audits are the first line of defense for a lot of businesses. They fill in the gaps before they become more serious problems. Additionally, they promote team accountability, making quality a shared responsibility.
External GMP Audits: A Closer Look
External audits provide an independent assessment of a company or its suppliers. They can be conducted by regulators, customers, or third-party experts offering GMP Auditing Services.
Objectives of External Audits:
- To verify compliance against local and international regulations.
- To ensure suppliers and partners meet contractual obligations.
- To demonstrate credibility to regulators and stakeholders.
- To provide impartial, expert-led evaluations.
Who Conducts External Audits:
- Regulatory authorities such as MHRA, EMA, or FDA.
- Third-party auditors engaged by the company.
- Customers evaluating their supply chain partners.
Benefits of External GMP Audits:
- Independent and objective findings.
- Assurance that systems meet global standards.
- Heightened confidence among supply chain participants.
- Supporting documentation for approvals, certifications, or inspections.
Because they affect regulatory credibility, supply chain agreements, and approvals, external audits frequently have higher stakes. For this reason, companies turn to expert GMP Auditing Services to prepare thoroughly.
Key Differences Between Internal and External Audits
Although the two forms of audits have the same ultimate purpose, they differ significantly in how they are undertaken, the focus they apply, and the results they yield.
Internal audits serve mainly as self-testing. They exist to provide businesses with an accurate representation of how their own mechanisms perform. Points to note are:
- Performed by in-house QA staff or trained personnel.
- Formatted more regularly and responsively, with opportunities for targeted review.
- Results typically result in corrective and preventive measures that enhance day-to-day operations.
- Lower-pressure environment that supports learning and improvement.
External audits, in contrast, are carried out by independent parties. These could be regulators, clients, or professional consultants. Their characteristics are:
- Frequently mandatory, associated with certification, supplier approvals, or regulatory audits.
- Results are more authoritative since they are evidenced by documentation of compliance.
- Compared to international best practices and industry benchmarks, not internal SOPs only.
- Results can directly influence licenses, authorizations, or supply contracts.
The distinction ultimately hinges on purpose and viewpoint: internal audits enrich internal processes, and external audits ensure compliance from the regulators’ and partners’ perspective. They are both essential, and together they give the fullest guarantee of quality.
When to Choose Internal vs. External Audits
Deciding between an internal or external audit isn’t just a procedural choice, it’s a strategic decision that affects compliance, supplier trust, and regulatory readiness.
Internal Audits Work Best When:
- Preparing for a regulatory inspection.
- Training teams on quality culture.
- Identifying gaps that may not be visible day to day.
- Monitoring ongoing compliance across different sites.
External Audits Are Essential When:
- Regulatory bodies conduct mandatory inspections.
- Qualifying new suppliers or contract manufacturers.
- Seeking to expand into new markets with stricter requirements.
- Providing documented proof of compliance to clients or partners.
The reality is not about choosing one or the other. The strongest compliance strategies use both internal and external audits in tandem. Internal audits keep systems healthy, while external audits provide the objective proof needed in a competitive market.
Common Challenges in GMP Auditing
Despite their importance, GMP audits are not without challenges.
Internal Audit Challenges:
- Limited resources and trained staff.
- Risk of bias if auditors are too close to processes.
- Audit fatigue when teams view audits as a burden.
External Audit Challenges:
- Suppliers resisting transparency.
- Uncertainty about the scope or depth of audits.
- Pressure of high-stakes findings that may affect certifications.
Addressing these challenges often requires outside support. Professional GMP Auditing Services provide the expertise, objectivity, and resources that internal teams sometimes lack.
The Role of Expert GMP Auditing Services
Pharmaceutical and biotech companies often rely on specialized consultants to strengthen their audit processes. By engaging expert GMP Auditing Services, organizations gain:
- Regulatory expertise: Consultants stay up to date with evolving guidelines.
- Independent insights: They provide objective assessments without internal bias.
- Global perspective: Their experience across markets strengthens compliance strategies.
- Specialized services: From supplier audits to temperature mapping, experts cover areas internal teams may overlook.
For companies in London and across the UK, Inglasia GMP Auditing Services in London provides trusted support. Their services extend beyond audits, including EU import and batch release, computer system validation, and quality management system projects.
Best Practices for Successful GMP Audits
Whether internal or external, certain best practices make audits more effective.
1. Maintain Readiness Year-Round
Audits should not trigger a rush to prepare. Instead, compliance should be part of daily operations, ensuring companies are always inspection-ready.
2. Train Staff Consistently
Employees must understand both procedures and the rationale behind them. Well-trained staff handle audits with confidence.
3. Use Audits as Opportunities
Rather than fearing findings, companies should use them to identify improvement areas. Each audit is a chance to strengthen systems.
4. Engage Professional Support
Bringing in expert GMP Auditing Services supplements internal capabilities and ensures no blind spots remain.
These practices create a proactive compliance culture, where audits become less about stress and more about growth.
Building a Strong Compliance Framework
Internal and external GMP audits may differ, but together they form the backbone of compliance. Internal audits strengthen everyday operations, while external audits provide the independent assurance regulators and partners require.
For quality and compliance leaders, the smartest approach is to integrate both types into a continuous improvement cycle. By doing so, companies not only meet regulatory standards but also protect patients, build trust, and gain competitive credibility.
Partner with Experts in GMP Auditing Services
If your company must enhance compliance, vet suppliers, or be ready for regulatory audits, having experts by your side is everything.
Inglasia GMP Auditing Services in London assists pharmaceutical and biotechnology firms with audits, EU batch release, validation projects, and quality system enhancements.
Trust Inglasia to lead your staff through internal and external audits with confidence, knowing your operations are of the highest GMP standards.
