Causes of Glass Particles in Injectable Medicines
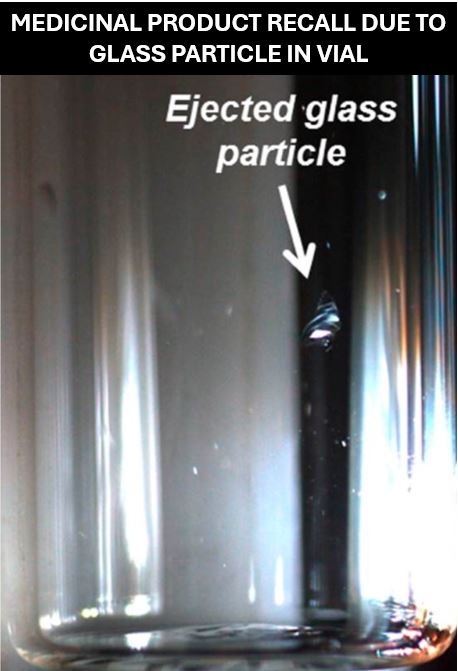
The FDA published a recall notice in October 2024 for 1 lot of Ascorbic Acid Solution for Injection vials to the user level due to glass particles in vial.
In some cases, glass particles can pass through the needle and may result in local irritation or swelling. The particulate matter could travel through and block blood vessels in the heart, lungs or brain which can cause stroke and even lead to death in extreme cases. Healthcare professionals must check these vials thoroughly before injecting the solution into patients.
The revised EU GMP Annex 1, effective from August 25, 2023, specifically addresses glass particles as a potential source of contamination and requires manufacturers to implement comprehensive controls through a Contamination Control Strategy (CCS). Glass particles in sterile medicine vials primarily result from two sources: mechanical damage during manufacturing and handling, and chemical degradation of the inner glass surface over time. These are issues that can arise at the vial supplier or the Pharmaceutical manufacturer.
Mechanical Causes
These issues generally occur during the high-speed filling, processing, and inspection stages in manufacturing lines.
- Glass-to-Glass Contact: Vials moving along production lines can slide against or impact each other, causing friction damage and chipping, which generates microscopic or visible glass fragments.
- Vial Breakage: Accidental breakage of a vial on the filling line can contaminate surrounding vials and equipment with glass fragments of various sizes.
- Ampoule Opening: For medicines packaged in glass ampoules (which are broken open for use), the action of snapping the top off a single ampoule can force glass micro-particles into the solution, a risk managed at the point of care, often using filter needles.
- Vial Manufacturing Defects: Flaws or stresses from the initial glass production or forming process (e.g., in the heel/bottom zone of a tubular glass vial) can create weak points that later result in particle formation.
Chemical Causes (Delamination)
Delamination is the flaking off of the inner glass surface due to a chemical interaction between the drug product and the container, often after a period of storage.
- Drug Formulation: Highly alkaline or certain buffer solutions (e.g., citrate and phosphate buffers) are more aggressive and can chemically attack the glass surface, causing the silicon oxide layers to leach and peel off as flakes (lamellae).
- Processing and Storage Conditions: Elevated temperatures during terminal sterilisation or long-term storage can accelerate the chemical interaction and degradation process. Long contact times between the solution and the glass surface increase the risk. Low fill volumes in a container can create an unfavourable surface-area-to-volume ratio, also increasing the propensity for the chemical attack that leads to delamination.
- Glass Composition and Treatment: The specific type of glass (e.g., Type I borosilicate glass is more resistant) and the manufacturing process used (e.g., flame-working tubular vials can create areas of reduced chemical durability) influence how susceptible a vial is to delamination.
Written by: Sanjay Nadarajah
