Medicines Recall Process – Overview
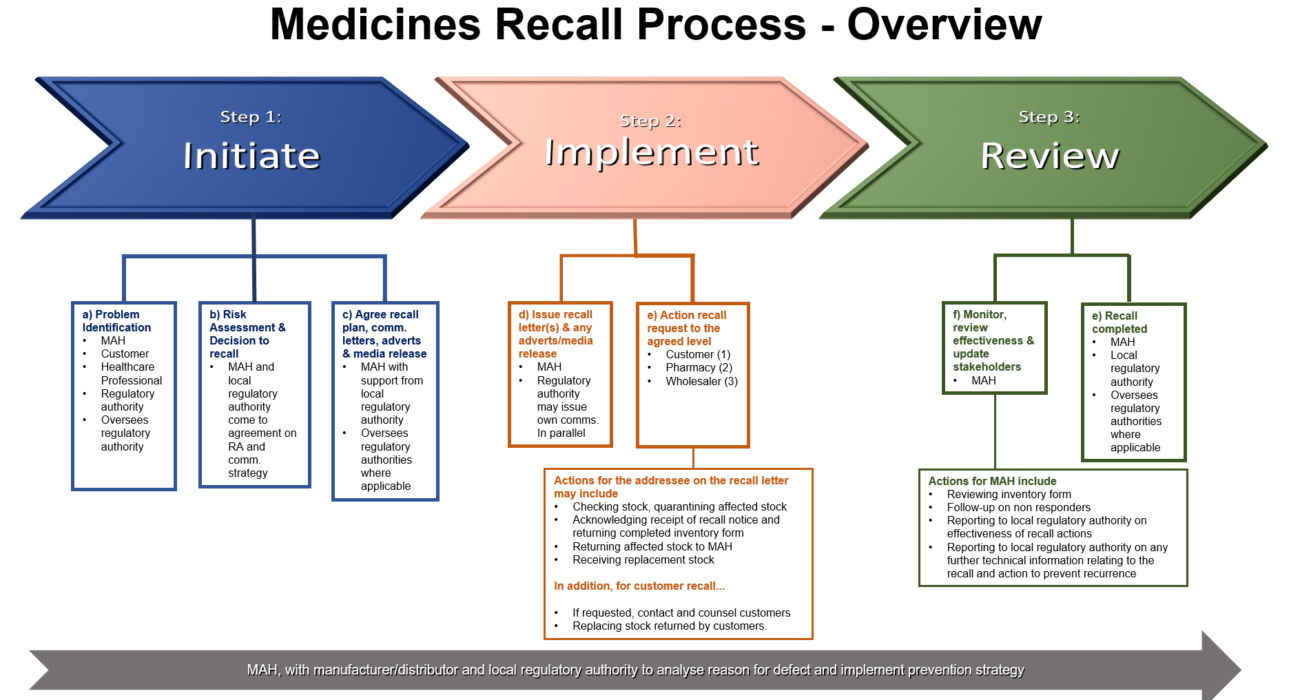
Departments and external stakeholders must work together to expedite recalls
The above process overview highlights the main considerations when executing a medicinal product recall. When a notification of a recall is received by the MAH (marketing authorisation holder in Country for the medicinal product), all departments within the organisation, external vendors and regulatory authority MUST priorities expediting any actions pertaining to the recall.
Recalls are lessons to learn from on the manufacturing, distribution and communication strategy of the organisation. If you require help with implementing a local or global level recall process, please reach out to us at info@inglasia.com
Written by: Inglasia Pharma Solutions
