Meeting rigorous GMP and GDP compliance requirements is not an option—it is the basis for trust in biotechnology and pharmaceutical supply chains.
Any temperature variation can potentially compromise patient safety, product quality, and your licence to practice. That’s why choosing the appropriate temperature mapping service matters.
At Inglasia Pharma Solutions, we combine regulatory expertise, principle-based consulting, and precision-guided delivery to give you a service not merely guaranteeing compliance but building confidence.
Arrange a Free ConsultationWhy Temperature Mapping Matters
During storage or transportation of medicinal products, small deviations in climate-controlled conditions can have serious impacts.
Regulatory authorities like the EMA, MHRA, and FDA demand evidence that your storage and distribution premises permanently remain within the specified parameters.
Temperature mapping demonstrates this by:
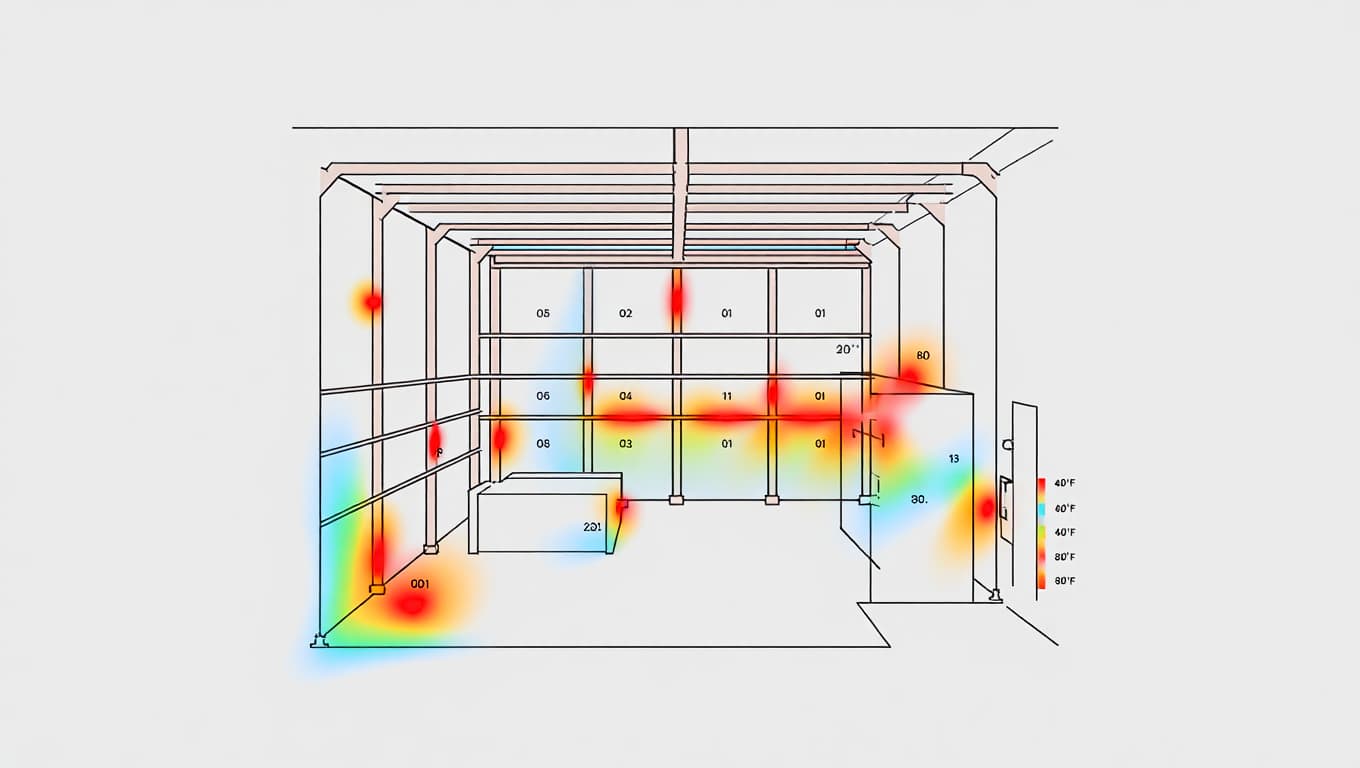
- Identifying hot and cold spots across storage areas.
- Ensuring that monitoring devices are accurately positioned.
- Providing documented evidence of compliance with GMP/GDP requirements.
Without mapping, you risk failed audits, compromised products, and reputational damage.
What Is Temperature Mapping?
Temperature mapping is a systematic study of how temperature and humidity behave across your controlled environment, be it a warehouse, cold room, freezer, vehicle, or container.
Using calibrated mapping sensors, data is collected over a defined period to simulate real-world conditions. The results guide decisions on where to install permanent monitoring probes and help confirm that your storage or transit conditions protect product integrity.
At Inglasia, we conduct accredited mapping studies designed to meet global standards, giving you assurance that your data withstands inspection scrutiny.
Who Needs Temperature Mapping Services?
Our temperature mapping service is built for organizations across the pharmaceutical supply chain, including:
Pharmaceutical Manufacturers
Validating storage and production facilities to ensure safe product handling.
Biotechnology Companies
Protecting sensitive biologics and advanced therapies from temperature excursions.
Global Distributors & Freight Forwarders
Demonstrating compliance during international transit and warehousing operations.
Clinical Trial Sponsors
Safeguarding investigational products across global clinical studies.
Whether you operate an ambient warehouse, a refrigerated storage area, or a temperature-controlled vehicle fleet, our service ensures your operations meet GMP, GDP, and international regulatory expectations.
Inglasia’s Approach to Temperature Mapping
Since 2011, Inglasia Pharma Solutions has delivered expert consultancy grounded in our seven pillars of Quality: Question, Understand, Accept, Liaise, Inform, Trust, You. This principle-driven foundation guides every mapping study we conduct.
Quality-First Methodology
Designed with inspection-readiness in mind, ensuring compliance and reliability.
Right Person, Right Task
Specialists assigned based on your facility type and regulatory requirements.
End-to-End Support
From study design and execution to data analysis and report generation, we handle it all.
Flexible Engagement Models
From one-off studies to fully outsourced Quality Departments, we adapt to your needs.
💡 Technology-backed: Our team works with leading mapping technologies, including Sensoscientific and Vaisala validation systems, ensuring accurate and validated data collection.
Key Steps in Our Temperature Mapping Service
Every project follows a structured, compliant process to ensure accurate, inspection-ready results.
Scoping & Study Design
We collaborate with your QA and operations teams to define parameters, including:
- Facility type (warehouse, fridge, freezer, vehicle)
- Duration of the study
- Seasonal considerations
- Regulatory requirements
Sensor Placement & Calibration
We deploy UKAS-calibrated sensors strategically across the environment, considering airflow patterns, storage layout, and regulatory expectations.
Data Collection
Sensors continuously record temperature and humidity over a set period, ensuring the dataset reflects normal operating conditions.
Data Analysis & Hot/Cold Spot Identification
Our experts analyze the dataset to identify risks such as uneven cooling, equipment malfunctions, or environmental vulnerabilities.
Final Mapping Report
You receive a comprehensive report including:
- Graphical data outputs
- Statistical analysis
- Identified hot/cold spots
- Recommendations for permanent monitoring placement
- Compliance confirmation against GMP/GDP requirements
This report is formatted to meet regulatory expectations and stand up to inspection scrutiny.
Unique Insights: Why Inglasia’s Mapping Service Stands Out
Most providers deliver data. We deliver data with meaning—actionable insights that tie back to compliance and patient safety.
- Inspection-Ready Documentation: Our mapping reports are written with regulators in mind, eliminating last-minute audit stress.
- Risk-Based Approach: We don’t just record data; we analyze risks across your supply chain, aligning with ICH Q9 quality risk management.
- Patient-Centric Focus: Every recommendation ties back to product integrity and patient safety, reinforcing your corporate responsibility.


Common Facilities We Map
Pharmaceutical products don’t all share the same storage needs, which is why our temperature mapping service covers a wide range of facilities. From bulk warehouses to compact freezers and temperature-controlled vehicles, we adapt each study to the environment at hand.
- Warehouses (ambient & refrigerated)
- Cold rooms and freezers
- Refrigerated vehicles and containers
- Controlled ambient transport systems
- Clinical trial depots and labs
Whether you need temperature validation for a new build or mapping calibration for existing facilities, our service covers it all.
Regulatory Alignment
We align each temperature mapping service with global regulatory frameworks, including:
- EU GMP Annex 15 – Qualification and validation requirements.
- EU GDP Guidelines – Storage and transport of medicinal products.
- WHO TRS 961 Annex 9 – Model guidance for storage/distribution.
- FDA 21 CFR Part 11 – Electronic records and data integrity.
By aligning with these standards, your business gains confidence in passing inspections from agencies like MHRA, EMA, FDA, and WHO PQ.
Temperature Mapping Reports: What You Can Expect
- Executive summary with compliance confirmation.
- Data sets with time-stamped sensor readings.
- Heat maps and graphical analysis of environmental behavior.
- Deviation logs with corrective action recommendations.
- Calibration certificates for deployed sensors.
This ensures full traceability and satisfies inspectors’ demands for clear, validated evidence.


Value Beyond Compliance
Compliance may be the baseline, but real value comes from operational insights:
- Identifying energy inefficiencies in climate control systems.
- Preventing product losses through early risk detection.
- Optimizing monitoring probe placement to reduce false alarms.
- Supporting continuous improvement in quality systems.
This dual benefit — regulatory compliance plus operational intelligence — is why clients return to Inglasia for ongoing mapping studies.
How long does a mapping study take?
Depending on facility type, a typical study lasts between 7 to 30 days, including planning, execution, analysis, and reporting.
Why Partner With Inglasia?
Working with Inglasia is working with an organization that has over a decade of successful GMP and GDP consultancy experience under its belt, relied upon by pharmaceutical and biotech industry leaders globally.
We offer the flexibility to help you with a single mapping study or be your outsourced Quality Department, as needed.
With a values-driven philosophy that prioritizes purity, transparency, and patient safety above everything, we strive beyond compliance to promote your reputation and defend your supply chain.
With Inglasia, you don’t just meet regulatory compliance — you build lasting trust with regulators, patients, and partners.
Next Steps
Whether you’re gearing up for an inspection, validating a new facility, or just enhancing your compliance stance, Inglasia’s temperature mapping service gives you the confidence you require.
Call us today to outline your project needs and let’s get your facilities and transport systems inspection-ready.
Frequently asked questions
Typically, facilities should conduct mapping at initial qualification, after significant changes (layout, HVAC upgrades, equipment changes), and periodically based on risk assessment.
Yes, provided the systems used meet 21 CFR Part 11 requirements for electronic records and data integrity.
Absolutely. Our consultants coordinate global mapping surveys, ensuring consistency across multinational operations.
We provide a corrective action plan aligned with your Quality Management System (QMS) to address risks and maintain compliance.
