Good Manufacturing Practice / Good Distribution Practice Vendor Management Process Flow
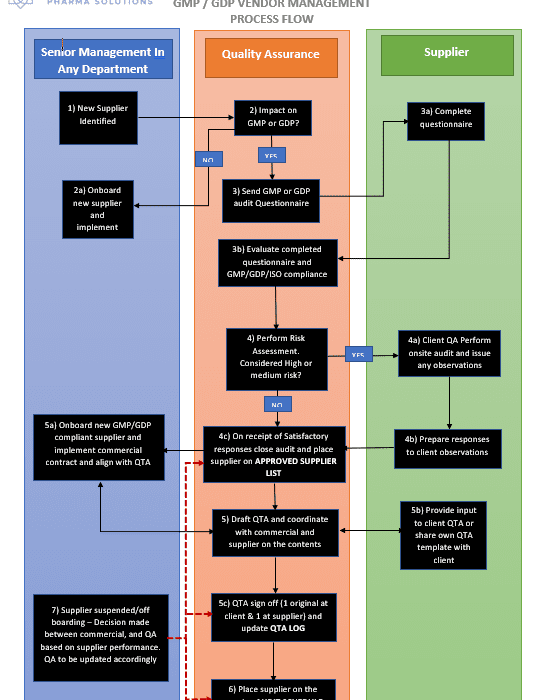
The process flow below provides simple guidance to anyone wishing to learn about how Pharma/Biotech suppliers should be managed where they have an impact on the medicinal product.
Any outsourced services provider, should be considered as an extension of your business and they must comply with your Quality System (QS) requirements for GMP and/or GDP. Wherever possible, for new vendors, it is advised that an initial onsite audit is performed to ensure they meet your QS requirements.
On satisfactory completion of the audit, implement a Quality Technical Agreement (QTA) with the vendor to ensure they comply with you Quality expectations that align with your QS.
Once approved, place this vendor on your approved supplier list and continually monitor their performance.
Written by: Sanjay Nadarajah, GMP/GDP Consultant
