Responsible Person (Import) – Requirements

Minimum checks required on medicinal product imported from an *EEA Country by a business in Great Britain (GB)
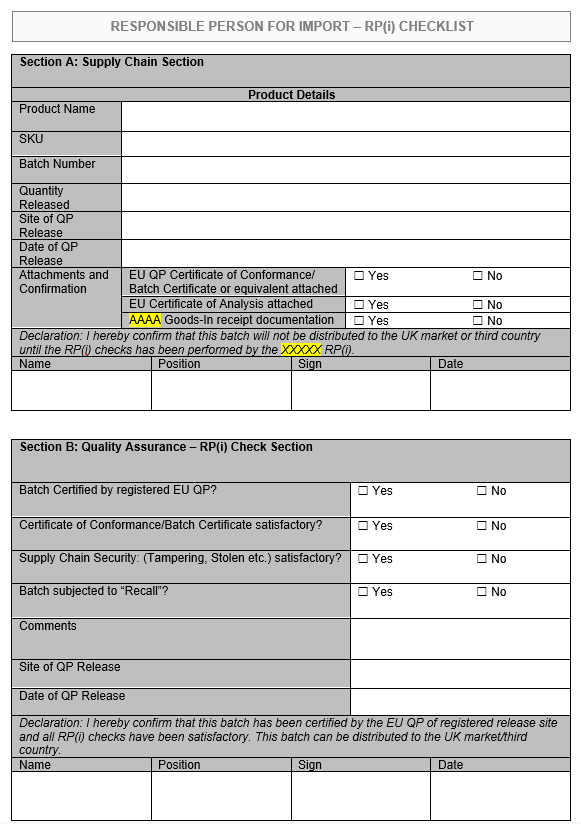
If your business in GB is looking to import medicinal products from the EEA, there are a number of checks which must be performed by the Responsible Person, prior to release for sale within GB or third country.
The below example form shows the checks which must be performed on each batch of medicinal product. These records must be retained in a Responsible Person (Import) GB release folder.
This applies to the following sourcing/supply models.
- A GB licensed medicine for use in GB
- A GB licensed medicine as an introduced medicine for supply to another third country
- An EEA licensed medicine – supply to fulfil special clinical needs
- An EEA licensed medicine imported as an introduced medicine for supply to another third country
- An EEA licensed medicine for use as starting material for a parallel import
*Link to the list of EEA Countries published on the MHRA website: https://www.gov.uk/government/publications/list-of-approved-countries-for-authorised-human-medicines
If you are in need of a Responsible Person, please contact us at info@inglasia.com to see how we can support your business.
Written by: Inglasia Pharma Solutions